Metabolism of Herbicides or Xenobiotics in Plants
Overview
This lesson will take an indepth view of how plants handle foreign chemicals (xenobiotics) such as herbicides. It will discuss the three main phases that plants use to handle toxic chemicals, which enzymes are involved in these biochemical conversions, how these processes help protect crops again phytotoxic chemicals and consider the importance of these processes to successful weed management.
Metabolism of Herbicides or Xenobiotics in Plants - Overview and Objectives
Tracy M. Sterling
Department of Entomology, Plant Pathology and Weed Science at New Mexico State University, USA
Deana M. Namuth
Department of Agronomy and Horticulture at University of Nebraska-Lincoln, USA
Scott J. Nissen
Department of Bioagricultural Sciences and Pest Management at Colorado State University, USA

 Lesson Navigation Tips: Lesson Navigation Tips:
- Click on ’Animations’ button found to the left in order to view the animation which supplements this lesson. You can also click on the animation icon within the text.
- Click once on figures to see enlarged versions.
- Click once on words in color to bring up their definitions.
|
Overview
Herbicide detoxication via metabolism is the primary mechanism of selectivity for most herbicides. Selective weed control using herbicides often relies on the ability of tolerant crop plants to detoxify the herbicide more rapidly than weeds. The same processes used by plants to detoxify herbicides are used for other xenobiotics (foreign chemicals); therefore, these terms will be used interchangeably. In this lesson, we will explore in detail three major phases of herbicide metabolism in plants, the enzymes involved in those processes and the final fate of herbicide metabolites. In addition, how crop injury or weed sensitivity might be altered by synergists, antagonists or other factors that affect metabolism will be described. Involvement of herbicide metabolism in resistance will also be discussed.
Objectives:
At the completion of this lesson, learners will be able to:
- Understand the importance of herbicide metabolism for crop tolerance (selectivity).
- Identify the three major phases of xenobiotic metabolism in plants.
- Explain the major enzyme systems involved during metabolism of xenobiotics in plants.
- Provide examples of each major phase and understand how metabolism may also lead to herbicide bio-activation.
Development of this lesson was supported in part by the Cooperative State Research, Education, & Extension Service, U.S. Dept of Agriculture under Agreement Number 00-34416-10368 administered by Cornell University and the
American Distance Education Consortium (ADEC).
Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. A contribution of the University of Nebraska Cooperative Extension, Lincoln NE 68583, Journal Series No. 1019.Metabolism of Herbicides or Xenobiotics in Plants - Introduction
All biological organisms have defense mechanisms to protect them from the negative effects of small quantities of foreign compounds (xenobiotics). These foreign compounds in plants include pesticides. Herbicides are a type of pesticide toxic to plants specifically, because they inhibit metabolic pathways unique to plants (e.g. photosynthesis). Herbicides are used successfully in weed management systems because they selectively harm sensitive weeds while leaving crops undamaged. Many factors contribute to successful herbicide action (e.g. successful placement or absorption and movement to target sites); however, the major reason herbicides are selective against weeds in crops is because crop plants are able to metabolize the herbicide to a non-toxic form. The basis for herbicide selectivity relies on enzymatic systems used in the plant’s normal metabolic processes.
The relative rates of herbicide absorption, translocation, and metabolism usually determine whether or not a herbicide will elicit a phytotoxic response. To better understand how these processes may each influence herbicidal action, they should be considered separately. Absorption has primary control over translocation, metabolism and phytotoxic action because the total amount of herbicide available for these processes is determined by the amount of herbicide absorbed by the plant. Metabolism influences both herbicide absorption and phytotoxic action. Metabolism generally converts the herbicide to a form with reduced phytotoxicity, thus, increasing the concentration gradient of the parent herbicide, so more is absorbed. Herbicide metabolism also influences phytotoxic action by either rendering the herbicide less or more active.
GENERAL VIEW OF THE MAJOR PHASES IN HERBICIDE METABOLISM -
Plants use a three-phase process to convert xenobiotics, such as herbicides or insecticides, into intermediates with reduced phytotoxicity. The ultimate result of these metabolic conversions is often movement of the altered xenobiotic into the vacuole of the plant cell or incorporation into cells walls.
These three phases are:
- Phase I - initial reactions such as oxidation, reduction or hydrolysis,
- Phase II - primary conjugation with endogenous substrates such as sugars, amino acids, or glutathione,
- Phase III - secondary conjugation, formation of insoluble residues or sequestration in the vacuole.
Click here to view the metabolism animation and see how a generic herbicide is altered through these three phases of metabolism.
| How does herbicide metabolism protect crops but not weeds from injury or death?
|
Rarely can plants mineralize a xenobiotic or completely oxidize it to CO2 like microorganisms can. On the other hand, animals and insects use only the first two phases, but and metabolism is usually faster and localized in an organ such as the liver or fat body; the metabolite is then eliminated from the body by excretion. Plants must alter these chemicals for long-term storage as non-toxic compounds because they are unable to excrete waste products.
Table 1 has detailed examples of metabolic conversions for each of the Phases. Click on the highlighted words to see specific examples of these metabolic processes. Written descriptions of processes are provided below.
TABLE 1 (modified from Shimabukuro, 1985)
| Herbicide Characteristics | Initial Herbicide Properties | Phase I Metabolism | Phase II Metabolism | Phase III Metabolism |
| |
Parent Compound |
- Hydrolysis
- Oxidation
- Reduction
|
Conjugation |
- Secondary Conjugation
- Incorporation into Cell Walls
- Vacuolar Sequestration
|
| Solubility |
Lipophilic (fat soluble) |
Some solubility in both lipid and lipid and aqueous conditions |
Hydrophilic (water soluble) |
Hydrophilic Insoluble Hydrophilic |
| Translocation |
Selective mobility |
Reduced mobility |
Limited or immobile |
Immobile |
| Herbicide activity |
Toxic to the plant |
Less toxic |
Minimal or nontoxic |
Nontoxic |
Metabolism of Herbicides or Xenobiotics in Plants - Phase I - Introduction
PHASE I
Phase I reactions can reduce or modify the phytotoxicity of herbicides, predispose the molecules to Phase II reactions and increase polarity. Therefore, Phase I reactions are considered the primary factor in selectivity. These reactions may also bioactivate the herbicide. Phase I reactions are not synthetic reactions in that the herbicide does not increase in size, but rather this phase is needed to introduce a reactive substituent group not already present. Some functional or reactive groups and their names follow (the hyphen indicates where the group attaches to the remainder of the molecule):
Amide = -CONH2
Amino = -NH2
Carboxylic acid = -COOH,
Cyano = -C=N,
Halogens (chloride, bromide, iodide, fluoride) = -Cl, -Br, -I, -F,
Hydroxyl = -OH,
Methoxy = -OCH3,
Methyl = -CH3,
Methylthio = -SCH3,
Sulfhydryl = -SH
The major reactions involved in Phase I conversions are oxidation, reduction or hydrolysis which form free amino, hydroxyl, or carboxylic acid groups. Many of the products from these reactions are acted on immediately by Phase II reactions as well.
Summary Note:
A Phase I reaction is the first step needed to make a herbicide less toxic; this reaction modifies the molecule so it is more water soluble and ready for Phase II and Phase III reactions which further detoxify the chemical. |
Metabolism of Herbicides or Xenobiotics in Plants - Phase I - Oxidation Reactions
Oxidation - (Figure 1) Oxidation reactions are some of the most common xenobiotic transformations occurring in plants.
 |
| Fig. 1: Oxidation |
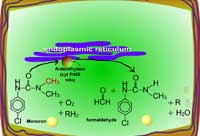
These Phase I reactions result in either detoxication or activation of the herbicide. Many of these oxidative reactions are presumed to be catalyzed by cytochrome P-450 monooxygenases. Monoxygenases catalyze reactions in which one of the two atoms of molecular oxygen is incorporated into the substrate (e.g. herbicide or xenobiotic) and the atom is reduced to water by an electron donor, such as NADPH (equation 1).
Equation 1:
X + RH2 + O2 → X-O + H2O + R
Where, X is the substrate (e.g. xenobiotic, herbicide), RH2 is the reduced cofactor such as NADPH, O2 is required as the second substrate, X-O is the oxygenated product and RH is the oxidized cofactor. Cytochrome P-450 monooxygenases are usually membrane bound and xenobiotic metabolism occurs in the endoplasmic reticulum.
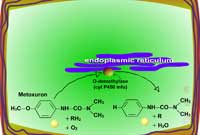
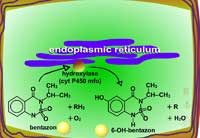
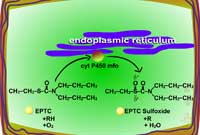
Examples of oxidation reactions include N-dealkylation or N-demethylation (Figure 2), O-dealkylation (Figure 3), aromatic hydroxylation (Figure 4), thioether oxidation (Figure 5) and β-oxidation (Figure 6).
 |
 |
 |
| Fig. 2: N-dealkylation or N-demethylation |
Fig. 3: O-dealkylation |
Fig. 4: Aromatic hydroxylation |
 |
 |
 |
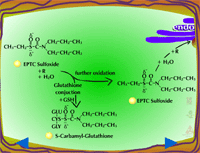
| Fig. 5a: Thioether oxidation - initially produces a sulfoxide, a molecule with less phytotoxicity |
Fig. 5b: Thioether oxidation - the sulfoxide can be conjugated with glutathione or further oxidized (Figure 5c) |
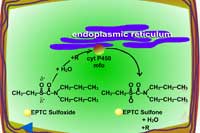
Fig. 5c: Thioether oxidation - the sulfoxide is converted to a sulfate, a molecule with less phytotoxicity |
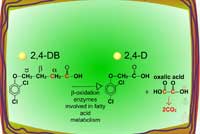 |
Fig. 6: β-oxidation
|
Some insecticides (organophosphates) can act as suicide substrates, irreversibly binding to cytochrome P450s in the plant and rendering the crop more sensitive to some herbicides (e.g. sulfonylureas). On the other hand, safeners or antagonists are able to induce (increase) cytochrome P450 monooxygenases in protected grass crops and therefore, enhance metabolism of some herbicides in the aryloxyphenoxypropionate, sulfonylurea, imidazolinone and sulfonamide families.
| Can inhibiting the enzymes that detoxify pesticides, alter the pesticide’s toxicity?
|
Metabolism of Herbicides or Xenobiotics in Plants - Phase I - Reduction Reactions
Reduction - (Figure 7)
 |
| Fig. 7: Reduction |
Reduction reactions as a means for metabolizing xenobiotics are fairly rare in plants. Also, these reactions are much less common Phase I reactions than the oxidation reactions. The most common reduction reaction in plants is aryl nitroreduction (Figure 8) where a nitro group on a phenyl ring is reduced to an amino group. These reactions are catalyzed by aryl nitroreductases which require a reductant such as NADPH. Other reduction reactions include deamination (Figure 9) where an amino group is removed or photochemical reduction of paraquat (Figure 10). Photochemical reduction of paraquat is nonenzymatic and is actually a form of bioactivation; an electron from the electron transport system in the chloroplast during the light reactions of photosynthesis reduces paraquat, creating a free-radical. This form of paraquat is very destructive to membranes because it uses its extra electron to reduce O2, creating superoxide free radical, O2-.. The free radical then causes lipid peroxidation (Click here to view an animation showing lipid peroxidation). This process is autocatalytic because once the reduced paraquat is oxidized or gives up its extra electron, it is again free to be reduced. Please refer to the Herbicides that Act Through Photosynthesis Lesson to see this in greater detail.
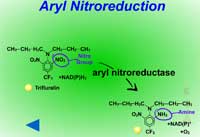
Fig. 8: Aryl nitroreduction |
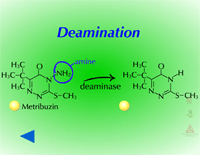
Fig. 9: Deamination |
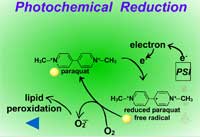
Fig. 10: Photochemical reduction |
Summary Note:
Reduction reactions add electrons and can either create a molecule with reduced toxicity or one with more activity. |
Metabolism of Herbicides or Xenobiotics in Plants - Phase I - Hydrolysis Reactions
Hydrolysis (Figure 11) - Hydrolysis reactions are common Phase I reactions in plants and involve herbicides possessing ester, amide or nitrile groups:
 |
| Fig. 11: Hydrolysis |
- Ester Hydrolysis (Figure 12) - Ester hydrolysis or de-esterification is the conversion of an ester, using water, into a carboxylic acid and an alcohol.
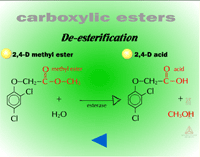 |
| Fig. 12: Ester hydrolysis |
De-esterifications during Phase I reactions are usually catalyzed by esterases, although nonenzymatic conversion can occur at pH 6.0. The esterases involved are somewhat characterized, but relatively little is known about which ones modify xenobiotics. Many of these enzymes are non-specific and reside in cuticles and cell walls. Herbicides applied as esters are fairly lipophilic and mobile in the cuticle; however, de-esterification is required prior to entry of the herbicide (now an acid) into the phloem via ion trapping. De-esterification will also increase or maintain the concentration gradient because the ester is converted into an acid and therefore, the gradient is steeper for entry of additional ester. De-esterification can be viewed as a form of bioactivation because the herbicide will not be translocated as readily in the ester form. In some cases, the de-esterified form of the herbicide is more toxic as well (i.e. fenoxyprop is more toxic to grassy weeds than fenoxyprop-ethyl).
- Amide Hydrolysis (Figure 13) - Amide hydrolysis is probably most important for the acylanilide group of herbicides. The major mechanism of selectivity for barnyardgrass control in rice using propanil, is that rice hydrolyzes propanil using the enzyme, acyl arylamidase. Barnyardgrass has about sixty-fold less acyl arylamidase in its tissue than rice does. Interestingly, some insecticides (carbamates and organic phosphates) act as synergists, by binding to the enzyme and inhibiting propanil hydrolysis resulting in more rice injury.
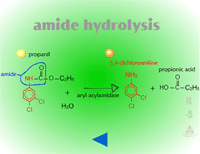 |
| Fig. 13: Amide hydrolysis |
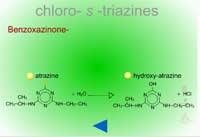
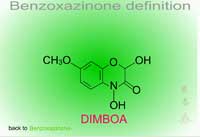
- Benzoxazinone-Mediated Hydrolysis of Chloro-S-triazines (Figure 14) - In roots of atrazine-tolerant corn, the natural product, DIMBOA (Figure 15) with water, performs a nucleophilic substitution at the Cl in position 2 of the triazine ring. This non-enzymatic reaction replaces the Cl with an OH at the same position resulting in hydroxyl-atrazine, a Phase I product with much less toxicity and which is now predisposed to Phase II reactions of conjugation.
 |
 |
| Fig. 14: Benzoxazinone-mediated hydrolysis of chloro-S-triazines |
Fig. 15: DIMBOA |
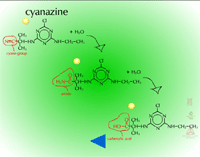
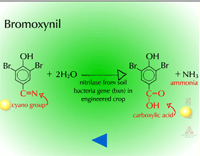
- Hydrolysis of Cyano Groups (Figure 16) - The cyano group (C=N) of herbicides such as cyanazine and bromoxynil can be metabolized via hydrolysis as well. The nitrile group is hydrolyzed to an amide and then to a carboxylic acid. The gene for the bacterial enzyme, nitrilase (Figure 17), has been moved from Klebsiella to crop plants to provide bromoxynil tolerance (e.g. cotton).
 |
 |
| Fig. 16: Hydrolysis of cyano groups |
Fig. 17: Nitrilase |
| Hydrolysis reactions require water. Which functional groups on pesticides are sensitive to hydrolysis reactions in plants?
|
Metabolism of Herbicides or Xenobiotics in Plants - Phase II - Introduction
PHASE II
Phase II reactions are also known as conjugation reactions. Conjugation reactions are anabolic processes which, in general, form compounds of higher molecular weight with greatly reduced biological activity, increased water solubility and usually reduced mobility. Major herbicide conjugates in plants are: simple and complex glucosides, glutathione conjugates and amino acid conjugates. Phase I metabolites of xenobiotics or those molecules which contain phenolic, N-arylamine, or carboxylic groups are rapidly conjugated in plants. In mammals, however, sulfate, glucuronic acid, and glutathione conjugates usually predominate. Although conjugates are excreted from animal systems, they remain in plant systems as conjugates or the conjugates are incorporated into insoluble polymers during Phase III reactions. Any toxicity remaining after Phase I is usually reduced further by Phase II reactions.
Metabolism of Herbicides or Xenobiotics in Plants - Phase II - Glutathione Conjugation
Glutathione Conjugation (Figure 18) - Glutathione conjugation involves the attachment of the small tripeptide, glutathione (γ-glutamylcysteinyl-ß-glycine) (Figure 19) , to herbicides containing halogen, phenolate or alkyl sulfoxide groups (Figure 20). On a herbicide molecule,
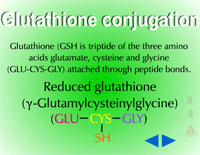 |
| Fig. 18: Glutathione conjugation |
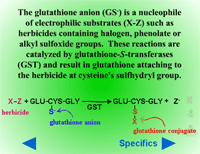
these groups can serve as leaving groups when reacted with the nucleophilic glutathione anion (GS-). In the reaction (equation 2), X is the herbicide and Z is the leaving group. These reactions are catalyzed by glutathione-S-transferases (GST). Conjugation proceeds as a nucleophilic displacement reaction with the glutathione anion (GS-) serving as the nucleophile. The sulfhydryl group (-SH) on the cysteine residue of the glutathione molecule is the reactive component required for this reaction. A simplified form of the reaction follows:
Equation 2:
X-Z + GS- → GS-X + Z-
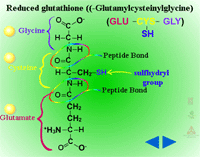 |
 |
| Fig. 19: Glutathione (γ-glutamylcysteinyl-ß-glycine) |
Fig. 20: Glutathione conjugation with herbicides (X) containing halogen, phenolate or alkyl sulfoxide groups (Z)
|
Pumps that recognize the glutathione conjugated with a xenobiotic (Figure 21) (GS-X) have been described in animal plasmamembranes and plant tonoplast membranes. These glutathione conjugates are thought to be transported into the plant vacuole (Figure 22). This sequestration is important to remove the glutathione conjugate from the cytoplasm for several reasons including the instability of glutathione conjugates. In some cases, vacuolar peptidases may cleave the glutamate and glycine of the original glutathione molecule, leaving the cysteinyl conjugate (cysteine-X) which may need further processing in Phase III reactions, such as incorporation into cell wall material.
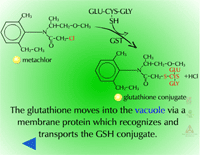 |
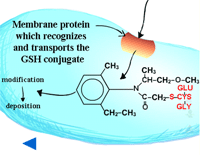 |
Fig. 21: Glutathione conjugated with a xenobiotic
|
Fig. 22: Transported into the plant vacuole |
Some safeners or antidotes protect corn, sorghum, and rice against soil-applied thiocarbamate and chloracetanilide herbicides by either increasing the levels of glutathione or by inducing activity of glutathione-dependent enzymes such as glutathione-S-transferase. This mechanism provides more crop protection while weeds remain susceptible.
Summary Note:
Crops can be protected from certain herbicides when safeners are applied to the crop because the safeners cause the crop to make more glutathione. Glutathione is attached to the herbicide in Phase II reactions to reduce herbicide toxicity. |
Metabolism of Herbicides or Xenobiotics in Plants - Phase II - Sugar Conjugation
Sugar Conjugation (Figure 23) - The most commonly detected herbicide-sugar conjugates are glucosides and glucose esters.
 |
| Fig. 23: Sugar conjugation |
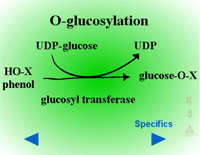
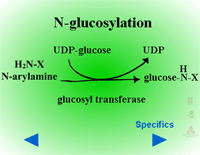
Herbicides containing phenolic, N-arylamine, or carboxylic acid groups as well as those metabolized to phenols, anilines or acids during Phase I can be made into sugar conjugates. Uridine diphosphoglucose (UDPG) is the most common sugar donor. The glucose attaches to phenol (O-glucosylation) (Figure 24a) or N-arylamine (N-glucosylation) (Figure 25a) groups via a ß-glucoside linkage. Specific examples can be seen in Figure 24b and 25b.
 |
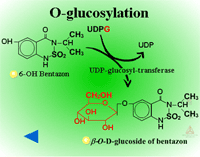 |
| Fig. 24a: O-glucosylation |
Fig. 24b: O-glucosylation |
 |
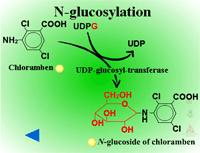 |
| Fig. 25a: N-glucosylation |
Fig. 25b: N-glucosylation |
Glucosyltransferases, which catalyze the conjugation reaction, are very specific for UDPG although these enzymes are thought to possess broad substrate specificity for xenobiotics. Broad substrate specificity may facilitate the glucosylation of herbicides in plants by these enzymes.
Glucose esters are formed in herbicides possessing acid groups (Figure 26a). Figure 26b shows a specific example where glucose is attached to 2,4-D at its carboxylic acid group. This conjugation product is not a stable detoxification; glucose esters can be converted readily back to active herbicides by cytoplasmic esterases (Figure 27).
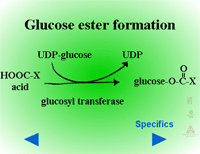
Fig. 26a: Glucose esters |
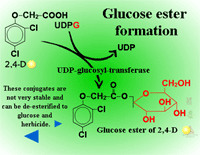
Fig. 26b: Esterification of
glucose to 2,4-D |
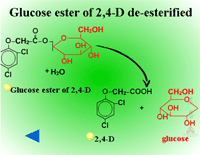
Fig. 27: Glucose ester of 2,4-D de-esterified |
Summary Note:
Glucose can be added to Phase I products to increase water solubility, reduce mobility, and reduce toxicity in a process termed ‘glucose conjugation’. |
Metabolism of Herbicides or Xenobiotics in Plants - Phase II- Amino Acid Conjugation
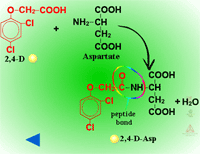
Amino Acid Conjugation (Figure 28) - Conjugation with amino acids is particularly common with the phenoxyacetic acid herbicides like 2,4-D. A peptide bond is formed between the herbicide’s carboxylic acid residue and the amino acid’s amino group. Some of the first evidence of amino acid conjugation with a herbicide was 2,4-D conjugation to aspartic acid (Figure 29). Other amino acids identified include glutamate, valine, leucine, phenylalanine and tryptophan. The most common amino acids conjugating with xenobiotics in plants are aspartate or glutamate. These conjugates can still be biologically active, but are relatively immobile. There is evidence that amino acid conjugates are excreted to the cell wall.
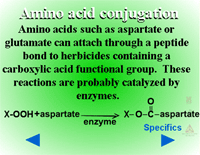 |
 |
| Fig. 28: Amino acid conjugation |
Fig. 29: 2,4-D conjugation to aspartic acid |
Metabolism of Herbicides or Xenobiotics in Plants - Phase III
PHASE III
Phase III reactions are unique to plants because plants do not excrete xenobiotics as animals do. Plants therefore, need to somehow remove the xenobiotic within their own system. Plants do this by either:
- conjugating Phase II products further
- moving metabolites to the vacuole for storage
- incorporating the metabolite into the cell wall region.
It is assumed that Phase III products are no longer toxic; however, this area of xenobiotic fate in plants is poorly understand, especially with reference to the identity of sequestered products and any subsequent fate in herbivores who consume the plants.
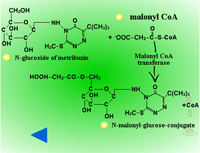
Secondary conjugation (Figure 30) - Herbicide glucosides can be further processed by the addition of a malonic acid residue to the sugar conjugate. In secondary conjugation or malonyl CoA conjugation, a malonic acid residue is attached to a product from Phase II sugar conjugation by an ester linkage (Figure 31). Malonyl CoA is the acyl donor (3 carbons will be added to the sugar conjugate) and the reaction is catalyzed by malonyl-CoA-transferases. Because malonyl conjugates are labile during extraction and purification, there may be more examples of secondary conjugation than currently identified in the literature. Malonylation may enhance vacuolar sequestration as well. When a xenobiotic is conjugated to glutathione, it can be catabolized to cysteine by the removal of the glutathione’s glutamate and glycine groups, prior to conjugation with malonate.
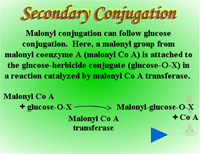 |
 |
| Fig. 30: Secondary Conjugation |
Fig. 31: Malonic acid residue is attached to a product from Phase II sugar conjugation
|
Insoluble residues - Pesticide metabolites may also be incorporated into cell wall material (Figure 32). These are known as insoluble or bound residues because they cannot be removed using conventional solvent extraction techniques. In general, terminal conjugation to insoluble or bound residues is not well understood, but studies have shown that between 1 and 70 % of the herbicide metabolite can be incorporated into structural components of the plant. This wide variation is thought to be due to differences in the xenobiotic, the plant species or tissue studied and the time of harvest. Xenobiotics sensitive to this fate usually contain aromatic or heterocyclic rings. An example of hydroxy-polychlorophenol (OH-PCP) incorporation into lignin is shown in Figure 33. Lignin is a polymer of phenolic groups. Peroxidases in the cell wall region create a free radical on the lignin molecule (at the highlighted -OH, for example) to which the OH-PCP can attach (Figure 33a). The PCP is essentially bound irreversibly.
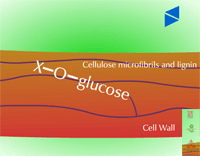
Fig. 32: A sugar conjugate of a pesticide (X) which has been bound to cell wall material |
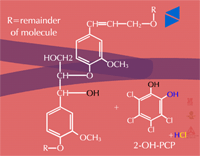
Fig. 33a: Lignin (left) and OH-PCP (right) in the cell wall regions |
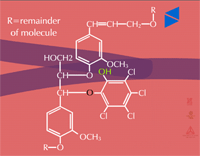
Fig. 33b: Insoluble residue of PCP |
Vacuolar sequestration (Figure 34) - Because plants store many toxic metabolites in their vacuoles and these toxic metabolites are released upon herbivore attack, it has long been thought that xenobiotic metabolites are moved into the vacuole. Recent evidence suggests there is a carrier on the vacuole membrane which recognizes glutathione conjugates (GS-X), transporting them into the vacuole (Figure 35). The carrier is dependent on ATP and apparently transports many glutathione conjugates (xenobiotics and natural products) into the vacuole. Glutathione and its conjugates are predominately anionic at the high pHs found in the cytoplasm of plant cells and therefore, rely on a transporter to move across the lipophilic membrane. To date, only a few glutathione conjugates have been shown to accumulate in plant vacuoles. No transporters of amino acids or glucose conjugates have been identified on vacuolar membranes. Malonylation may aid in vacuolar sequestration as well.
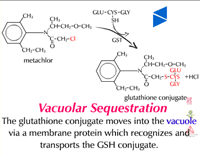 |
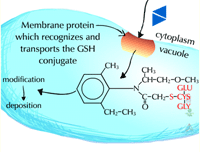 |
| Fig. 34: Vacuolar sequestration |
Fig. 35: A glutathione conjugate (GS-X) being transported into the vacuole
|
| Name three ways plants deal with pesticide metabolites in Phase III reactions, instead of excreting the pesticide metabolite like animals do.
|
Metabolism of Herbicides or Xenobiotics in Plants - Field Considerations
Field considerations of Herbicide Metabolism
Differential metabolism of herbicides between crops and weeds has long been the basis for successful weed management. Selectivity can be altered by a variety of factors that can increase or decrease herbicide activity, including interaction with soil-applied insecticides, use of safeners, or the development of resistance in weeds. Bioactivation is also an example of selectivity. This section provides some examples of how important herbicide metabolism is in crop protection.
Synergists -
Corn root-worm insecticides and sulfonylureas - The root-worm insecticide, terbufos (an organophosphate insecticide), is applied as an in-furrow treatment with corn seeds. This treatment enhances the activity of primsulfuron and thus, corn injury. Crop safety is lost because terbufos binds to cytochrome P450 enzymes in corn, so the crop is less able to detoxify the herbicide. To avoid this problem, growers were advised not to use an in-furrow type treatment or to use a new formulation of Counter called Counter CR (Controlled Release). Now growers can also use BT-resistant crops.
Insecticides and propanil - Propanil activity on rice weeds can be increased with the addition of malathion (an organophosphate insecticide). Organophosphate insecticides can inhibit acyl arylamidase, the enzyme responsible for propanil metabolism. This approach also increases the likelihood of crop injury if too much enzyme inhibition occurs in the crop as well.
Metribuzin and PABA - Metribuzin activity on ivyleaf morning glory (Ipomoea hederacea) can be increased when the synergist PABA (picolinic acid t-butylamide or MZH 2091) is included in the spray solution. Normally, ivyleaf morningglory is able to metabolize metribuzin via deamination followed by conjugation. However, in the presence of PABA, deamination is slowed and thus, ivyleaf morningglory is more susceptible to metribuzin.
Antagonists (also known as safeners, antidotes or crop protectants) - Safeners selectively protect crop plants from herbicide injury without protecting weeds. Many safeners are structurally similar to the herbicides that they antagonize.
Soil-applied safeners - These safeners are applied with the seed prior to planting or applied to the soil or crop together with the herbicides. They protect large-seeded grass crops (corn, sorghum, rice) against pre-plant incorporated or pre-emergence applications of thiocarbamate and chloroacetanilide herbicides. Most of the evidence suggests that these safeners protect the crop from injury by inducing enzymes that metabolize the herbicide, rather than by affecting herbicide absorption and translocation or its site of action. For example, these safeners increase the level of glutathione conjugation of chloracetanilide and thiocarbamate herbicides by inducing glutathione-S-transferases. As a result they may also increase the level of glutathione in the plant tissue.
Foliar-applied safeners - These safeners enhance the activity of cytochrome P450 monooxygenases and glucosyltransferases to protect grass crops from aryloxyphenoypropionate, sulfonylurea or imidazolinone herbicide injury. An example is the safener, fenchlorazole-ethyl used to protect grass crops (i.e. wheat, rye, barley) from fenoxyprop-ethyl injury. Normally, this class of herbicides controls grasses, but not broadleaf plants. These crop species possess higher levels of glutathione than grassy weeds and the safener enhances these levels further in the crop species, protecting them from herbicide damage.
Bioactivation
In some cases, the biological activity of certain herbicides is increased via metabolism. A classic example is the bioactivation of the phenoxybutyric acids (e.g. 2,4-DB). These herbicides are not toxic until two carbons are removed enzymatically from butyric acid via ß-oxidation (Figure 36). This selectivity mechanism protects legumes from herbicidal injury as they do not catalyze this reaction; broadleaf weeds cleaving off the two carbons convert the phenoxybutyric acid into the herbicidal phenoxyacetic acid, such as 2,4-D.
Another example of bioactivation is the photochemical reduction of paraquat (Figure 37). This reaction is nonenzymatic as an electron from the electron transport system in the chloroplast during the light reactions of photosynthesis reduces paraquat, creating a free radical (see the Reduction Reactions section for further explanation).
 |
 |
| Fig. 36: ß-oxidation |
Fig. 37: Photochemical reduction |
Herbicide resistance
Mechanisms of herbicide resistance are often based on changes in site of action; single point mutations in the genetic code alter the gene product such that it no longer binds the herbicide. There are several instances where the mechanism of herbicide resistance is metabolism based. In these cases, the resistant plant is able to metabolize the herbicide to a non-toxic form. Examples of enzymes expressed in resistant weeds that detoxify the herbicide include cytochrome-P450 monooxygenases, acyl arylamidases, and glutathione-S-transferases. An example of metabolism-based selectivity is Liberty Link crop technology. Crop resistance to Liberty (glufosinate) is based on the ability to metabolize the herbicide. Herbicide Resistance Lesson for more detail.
Other examples where herbicide metabolism may affect selectivity:
 |
| Figure 38: Corn response to soil applied Balance under warm soil (left) and cool soil (right). |
The formulated product, Distinct® is a combination of the auxinic herbicide, dicamba and the auxin transport inhibitor, diflufenzopyr. Diflufenzopyr enhances dicamba activity on broadleaf weeds because it blocks the transporters that would normally move auxin from cell to cell; because broadleaf weeds are less able to metabolize dicamba, the trapped herbicide is phytotoxic. If this formulation is applied when corn plants are too young, there is risk of crop injury. The younger corn tissue is apparently unable to handle dicamba accumulation. Is it possible they are not able to metabolize dicamba at a fast enough rate?
Crop injury can increase under cool, wet conditions (Figure 38) - is it possible that the herbicide is taken up and translocated in these plants, but mechanisms of herbicide metabolism are compromised?
Metabolism of Herbicides or Xenobiotics in Plants - Summary
Herbicide metabolism is the primary mechanism of selectivity where the desirable plant is not injured because it has rendered the herbicide less toxic and the undesirable weed dies because the herbicide stays in its active form. Herbicide or xenobiotic metabolism proceeds in plants through a three phase process. The first phase introduces reactive groups which then can be acted on by Phase II reactions. Phase II reactions usually increase the size and polarity of the molecule by adding either glucose or glutathione. Under Phase III reactions which are unique to plants because they cannot excrete metabolites, the plant either stores the xenobiotic metabolite in the vacuole or incorporates it into cell wall materials. Metabolism-based selectivity is also important in herbicide resistance and can be altered through the addition of synergists or antagonists.
REFERENCES
Hall, J. C., R. E. Hoagland, and R. M. Zablotowicz, editors. 2001. Pesticide Biotransformation in Plants and Microorganisms - Similarities and Divergences. ACS series 777. American Chemical Society, Washington DC. 432 pp.
Hatzios, K. K., ed. 1997. Regulation of Enzymatic Systems Detoxifying Xenobiotics in Plants. NATO ASI Series 3. High Technology - Vol. 37, Kluwer Academic Publishers, Boston, 385 pp.
Kreuz, K., R. Tommasini, and E. Martinoia. 1996. Old enzymes for a new job: Herbicide detoxification in plants. Plant Physiol. 111:349-353.
Sandermann, H. 1992. Plant metabolism of xenobiotics. TIBS 17:82-84.
Shimabukuro, R.H. 1985. Detoxication of herbicides, In (S.O. Duke, ed.) Weed Physiology, Vol. II, pp. 216-240.
 Lesson Navigation Tips:
Lesson Navigation Tips:








































