
This lesson contains information about the history, life cycle, and host plants of the European corn borer and information relating to the history and biology of Bacillus thuringiensis.
 |
 Lesson Navigation Tips: Lesson Navigation Tips:- Click on ’Animations’ button found to the left in order to view the animation which supplements this lesson. You can also click on the animation icon within the text. - Click once on figures to see enlarged versions. - Click once on words in color to bring up their definitions. - This lesson is approximately 14 pages long when printed. - Takes most learners 15-60 minutes to complete. |
"The European corn borer, Ostrinia nubilalis, came to North America in broom corn (used to make brooms) imported from Hungary and Italy in the early 1900?s. Broom corn is related to shattercane and sorghum (Sorghum bicolor L.). The European corn borer (ECB) was first noticed around Boston, Massachusetts in 1917. ECB infestation gradually moved westward and by 1939 infestation of the Corn belt had begun" (Mason et al, 1996).
 |
The life cycle of the European corn borer involves four main stages: egg, larva, pupa, and adult. The borer is representative of the larva stage (Fig. 1) and the ECB moth is representative of the adult (Fig. 2). Together, these four different stages of development make up one generation.
The mating process for the first generation of ECB begins in the spring (approximately May to July depending on geographic location). The moths will begin to emerge from diapause and begin mating. The moths find an action site consisting mainly of grasses near the corn field. This area provides water in the form of dew, which is needed for the female moths to emit an attraction pheromone to the males.

Fig. 1 ECB: First instar larvae (Jim Kalisch, University of Nebraska Lincoln. 1995)
Fig. 2 ECB: Adult female moth (Jim Kalisch, University of Nebraska Lincoln. 1995)
The female moth will begin laying eggs in June, after sunset when the wind is calm and the temperature is warm. 'The eggs are laid in masses on the underside of corn leaves near the midvein' (Michigan State University, 1997). 'The masses are approximately ¼ inch in diameter and made up of 15-30 individual eggs (Fig. 3).
Each female moth is capable of producing two egg masses per night for 10 nights. However, most of the eggs are laid in the first six nights' (Mason et al, 1996). The egg masses appear white when first laid, but as the eggs mature they will turn a pale yellow and then translucent at the blackhead stage. The blackhead stage occurs right before hatching when the larvae are visible through the translucent egg masses (Fig. 4). The black centers of the eggs are caused by the black heads of each individual larva. 'The egg masses will hatch three to seven days after being laid, depending upon the temperature and other weather conditions' (Mason et al, 1996).

Fig. 3 ECB egg mass: This picture shows the egg mass after being laid near the midvein of the corn leaf. (Jim Kalisch, University of Nebraska Lincoln. 1995)
Once the ECB eggs have hatched, the larval stage of development begins. The emerging larvae move from the underside of the leaves to the center of the plant, the whorl, for shelter and food. During the larval stage there are five developmental stages called instars. Throughout these five instars the larva are growing and preparing to become adults.
At the first instar the larvae are white in color with a black head and are approximately 1/16 inch long. During the second instar, the ECB grows larger and becomes more yellow in color. Both of these two instars feed on the leaf tissue and create holes in the leaves of the whorl. However, the second instar creates larger holes than the first. 'ECB feeding in the whorl lasts approximately two weeks'(Witkowski and Wright, 1997).

Fig. 4 ECB blackhead egg mass: This picture shows the egg mass in the blackhead stage, prior to the larvae emerging. (Jim Kalisch, University of Nebraska Lincoln. 1995)
The third instar begins feeding on the outside before boring into the stalk and creating cavities in the plant. The fourth and fifth instars feed only on the stalk. Once the larvae have reached the fifth instar, their color is grayer with brown spots, outlined in black, present on each section of its body. At the fifth instar, the larvae are approximately one inch in length. The ECB larvae will live and feed inside the stalk.
The second generation of ECB is much like the first, however there are some slight differences. In the second generation, the larvae do not begin feeding on the stalk of the corn plant until the fourth instar, due to the hardness of the maturing stalks. In the first generation the stalks are tender and can be infested more easily by the third instar. As the corn plant reaches maturity the tassel emerges from the whorl of the plant thus eliminating second generation larval feeding on this part of the plant. The second generation larvae tend to feed on the leaf axil, closer to the stalk, rather than the blade of the leaf. The larvae also consume pollen that has collected in the leaf axil.
In certain regions, of the southern United States, there is a third and possibly a fourth generation of ECB. These develop in the same manner as the previous generations. However, the fifth instar must be present in the corn stalk or plant debris in order for diapause to occur after the final generation.
 |
| Fig. 5 ECB pupa: This picture shows the pupa in a corn stalk, prior to moth emergence. (David L. Keith, University of Nebraska Lincoln. 1971) |
Sweet corn, popcorn, and seed corn are the second most affected plants by ECB, behind field corn. Other crop species that are occasionally infested include, cotton, peppers, snap beans, wheat, and potatoes. Some plants that have been reported to be infested with ECB but cannot host the larva through their lifecycle include 'apples, chrysanthemum, lima bean, soybean, black-eyed pea, small grains, sorghum, tomato, onion, and sage' (Mason et al, 1996).
Damage
Damage caused by European corn borer ranges from mild to severe. Since corn is the first choice of a host for the ECB, damage to field corn, sweet corn, popcorn, and seed corn is usually the most severe. The ECB attacks many different areas of the plant. The first generation of ECB attacks the leaves and stalks of the corn plant. ECB larvae leave small holes in the leaves and holes entering the stalk of the plant indicating the beginning of a tunnel. The second generation ECB feed on the leaves, the stalk, the ear, and the bottom portion of the leaves closest to the stalk called the leaf collar and sheath. The third generation continues to feed on the leaf collar and sheath, as well as the ear (Fig. 6a and 6b).
Any of the generations that feed on the corn plant can cause problems both while the plant is growing and during harvest. Feeding on the leaves and the stalk disrupt the biological processes of the plant (Fig. 7).
For example, when the ECB larvae damage the green leaves of the plant, this reduces the plant’s ability to perform photosynthesis. When the stalk of the plant is damaged, this reduces the amount of water and nutrients that can travel through the stalk to the developing ear. Therefore, since the amount of nutrients is reduced, the developing ear cannot reach its full potential, which results in a reduced yield for the producer. European corn borers also feed on the ear shank of the corn plant. Once the ear shank is damaged this increases the probability that the ear will drop to the ground, making it unable to be harvested.

Fig. 7 ECB 5th instar larva: This picture shows the ECB damage done to the inside of a corn stalk. (David L. Keith, University of Nebraska Lincoln. 1977)
When damage occurs from ECB the plant is also at a higher risk for disease. Injury caused by the ECB, creates an easy location for disease pathogens to enter the plant. In this situation, stalk rot is a common disease to affect corn plants. When disease and damage occur, the stalk weakens and complicates the harvest process. Plants that have become lodged due to disease or damage, are often unable to be harvested and may even make it difficult for equipment to move through the field.
Sweet corn, popcorn, and seed corn are affected in much the same way as field corn. Since sweet corn is sold fresh and for canning, ear feeding by ECB is the most damaging. However, in popcorn shank feeding by ECB affects the plants, causing the ears to drop on the ground and decreasing harvestable yields. European corn borer feeding on seed corn plants can directly affect the seed stock for the next growing season by reducing the amount of seed that is produced per plant.
Damage that occurs to other types of crops, such as peppers or potatoes, includes boring into the fruit and stalk and laying eggs on the leaves of the plant. This is a problem because the moths will lay their egg masses on other types of plant material. Once these eggs are mature they will hatch and larval feeding causes damage to both the leaves and fruit. The damage on the leaves will reduce the plants ability to grow normally. This can directly affect the production of fruits and vegetables. ECB will also bore into fruit making it unsuitable in the fresh market. Since the European corn borer is a damaging pest, it needs to be controlled to avoid excessive crop damage.
Now let us look at a control method farmers have used to combat the adverse affects of European corn borers. Bacillus thuringiensis (Bt) is a naturally occurring, soil borne organism. Bt was first discovered in a diseased silkworm colony in 1901 by Ishiwata, a Japanese bacteriologist. However, not much research was done until 1911 when a German bacteriologist, Berliner, took interest in this bacterium (Reardon et al, 1994).
Bacillus thuringiensis can vary in size, ranging from a small bacterium, measuring 0.5 x 1.2 µm (micrometers) to quite large at 2.5 x 10 µm. The Bt cells are rod shaped and can occur as single cells or in chains (Holt, 1984). The bacteria are so small that approximately 8,000 Bt cells can fit on a circle the size of the period at the end of this sentence. (This number was calculated by figuring the total area of a single Bt bacteria (2.5 x 10 µm) and comparing that to the total area of a period on a page.)
Once Bt was discovered and cultured, researchers found that this bacterium produced a toxic crystalline protein lethal to certain insects. The insect must ingest the toxic protein produced by Bt in order for death to occur. The Bt protein will kill Coleoptera (beetles), Lepidoptera (moths, butterflies, and caterpillars), and Diptera (flies and mosquitoes). However, there are many different strains of Bt with Bt proteins specifically lethal to certain insects. For instance, protein from a certain strain of Bt is lethal only to insects in the order Lepidoptera. Another protein is lethal only to the order Coleoptera. (See table 1,Hoffmann and Frodsham, 1993.)
|
|
|
| Colorado potato beetle and elm leaf beetle adults and larvae | |
| Colorado potato beetle and elm leaf beetle adults and larvae | |
| caterpillars, such as ECB, cutworms, and cabbageworms | |
| mosquitoes, blackflies, and fungus gnat larvae | |
| wax moth larvae and various caterpillars, especially diamondback moths |
The Bt toxin that kills the ECB is also known as a crystal protein. This crystal protein is made in the bacteria cell and as the cell goes through its lifecycle, the crystal protein is exuded. The bacteria then develop into spores and the crystal protein is attached to the spore (Fig. 8). In the case of genetically engineered plants, the genetic code for the crystal protein is put into the plant. Therefore, when the plant tissue is ingested by the insect, only the crystal protein is eaten. On the other hand, when Bt is used as a spray applied insecticide, the entire spore and crystal protein is ingested. This is the same way ingestion occurs in nature (Madigan et al, 2000). Ingestion of the crystal protein, by the ECB, must occur in order for death to occur.
The crystal protein is chemically activated within the larval mid-gut and becomes a toxin. The crystal protein can only be activated in a mid-gut environment with a pH of 9.5 (Deacon, 2001). which is characteristic of the ECB. 'The toxins bind to cells within the gut and cause leakage of cell fluid and then lysis to occur' (Madigan et al, 2000). These crystalline toxins paralyze the digestive tract of the ECB larvae and cause them to stop eating. The crystalline toxins are labeled “CRY” with a number representing order of discovery (i.e.: CRY 1 was the first CRY protein to be characterized). Affected larva become inactive, stop feeding, their body becomes flaccid and the head may appear overly large for the body of the larva (Hoffmann and Frodsham, 1993). The larvae stop eating and die from 12 hours to 5 days later. However, this depends upon the size of the larvae and how much of the Bt toxin was eaten (Willie, 2001). To see how Bt causes death in the ECB larva, click the animation below.
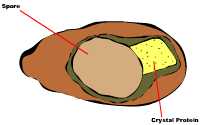
Fig. 8: Spore and crystal protein (Madigan et al, 2000)

The application of Bt crystal proteins as a biological insecticide was first introduced in 1938 in France. The technology was first used in the U.S. in the 1950’s (Deacon, 2001). There are many advantages to using Bacillus thuringiensis as an insecticide, including few known hazards to humans and animals. Applying insecticidal Bacillus thuringiensis can be done up to harvest, creating a larger application window. Once the biological insecticide has been applied, re-entering the field can be immediate (Willie, 2001).

Fig. 9 Shows a sprayer applying chemical across a field. (UNL, 2001)
Bacillus thuringiensis, as a bacterial insecticide, can be applied with conventional spraying equipment. An example of spraying equipment can be seen in Figure 9.
Since it is required that the ECB ingest the Bt protein to be killed, it is important for the spray equipment to have good coverage over the entire field. Bt must also be applied when the population of larvae is at the highest level since this type of organic chemical will only kill the insect in the larval stage. Also, organic insecticides containing Bacillus thuringiensis are only effective during the first generation of ECB (1Wright et al, 1994). This is due to the coverage of the spray application across the field and how long the insecticide remains active. Some of the most common organic insecticides containing Bt used in Nebraska include, Dipel® and Xentari® Biological insecticides. Both Dipel and Xentari can be used on field corn, sweet corn, and popcorn, but only Dipel can be applied to seed corn fields. Dipel can also be used on cotton, apples, beans, potatoes, soybeans, and peas to name a few. Xentari can be applied to peppers, peas, legumes, potatoes, soybeans, apples, and cotton as well as many others (Kelly, 2004).
The application of Bt cultures as an insecticide has the advantage of safety and organic acceptibility. Effective application and timing limits its ability to control economic damage.
The advent of genetic engineering technology has provided scientists with the capability of developing plants with the ability to make this bacterial protein in the desired plant tissues. In the late 1980’s, scientists were able to isolate and clone the gene coding for one of the Bt proteins toxic to ECB. By 1996 the first Bt corn hybrid was on the market (Witkowski et al, 1997). Once the Bt gene was successfully inserted into corn the level of toxicity to insects increased making the final expression of the toxic crystalline protein higher than the level in Bt insecticide sprays. There are five different Bt CRY genes that have been inserted into corn grown commercially. (See Table 2, National Corn Growers Association, 2005 and 2Wright et al, 2000)
|
Event |
Gene Coding Region |
Promoter | Characteristics | US EPA Approval (as of 2001)** | Trade Name |
|
Mon 810 |
Bt expressed in all plant tissues, controls both 1st and 2nd generation ECB | ||||
|
Bt-11 |
CRY 1Ab |
CaMV 35S | Bt expressed in all plant tissues, controls both 1st and 2nd generation ECB, glufosinate herbicide tolerance | food and livestock feed | YieldGard |
|
E-176 |
Bt expressed in only green plant tissue and pollen, controls both 1st and 2nd generation ECB | ||||
|
DBT-418 |
Bt expressed at some level in all plant tissues, controls 1st generation ECB | ||||
|
TC1507 |
Bt expressed at some level in all plant tissues, controls 1st and 2nd generation ECB, glufosinate herbicide tolerance | ||||
|
CBH-351 |
different protein from 1A, Bt expressed in all plant tissue, controls 1st and 2nd generation ECB |
The advantage of these biotechnology improved crops is they now have resistance to ECB without the need of applied chemicals. However, at the same time, this technology has been the center of debate. There are opponents that question the ethics, food safety, and environmental impact of these crops. (Fig. 10)
Ethically, the practice of inserting genes from one organism into a completely unrelated organism (i.e. a gene from an animal into a plant) has raised concern with some. (Schneider, 2002) Bt corn is an example of this practice where a gene from one organism (a soil bacterium) was inserted into another (a corn plant). Is it right to change the way organisms were naturally created? What will be the long term effects of putting foreign DNA material into the food supply? How will it affect feed for livestock? These are just three questions often asked by apprehensive consumers related to Bt corn and genetically engineered products.
A question related to the ethics of planting Bt corn revolves around who really benefits from growing this product. Is it the small family farms or the large farms? In a survey conducted by the University of Minnesota the majority of the farmers who responded stated that biotechnology would benefit large farms. (Ramirez-Rocha and Hurley, 2004) What is the reason for this response? This could be attributed to the added cost associated with purchasing biotechnology enhanced seed or to the reduction in labor and chemicals seen as a result of using this new technology.
Food safety is another issue related to genetic engineering. StarLink corn, which at the time had been approved for animal feed, mistakenly found its way into U.S. food chains before being approved by regulatory agencies for human consumption. In this case, the specific CRY protein had shown potential for allergenicity due to its longer digestion times observed in the laboratory, a characteristic of some known allergens. Therefore, until further research had been conducted on the effects in humans, the product was released for animal feed only, which in the end was a mistake due to the difficulty in keeping grains separated.
 |
| Fig. 10: Example of a corn field under center pivot irrigation. (UNL, 2004) |
European corn borer has been a threat to crops in the United States since the early 1900’s. Controlling ECB was made possible with the use of Bt cultures as insecticides. Recently, genetic engineering has allowed Bt genes to be inserted into plants so they can produce their own Bt insecticidal protein.
There are four stages in the ECB lifecycle including egg, larva (also instars), pupa, and adult. The stage that causes the most damage to corn is the larva stage. The main damage caused by ECB to corn plants includes holes in the leaves and cavities inside the stalk.
European corn borer have a variety of host plants. Some of the most common include corn, cotton, vegetables, and soybeans. The ECB also cycle through different generations. There can be anywhere from one to four different generations depending on what part of the United States the ECB are located.
Bacillus thuringiensis is a soil borne organism, with many different strains found throughout the world. The most commonly susceptible insects are coleoptera, lepidoptera, and diptera. Bt kills ECB by being ingested. Once the Bt toxin is ingested, it will bind to the mid-gut releasing the toxin and causing death.
Currently, there are three different events (Mon 810, Bt-11, and TC1507) commercially available in transgenic corn, each with its own characteristics. These characteristics can include expressing Bt in the green leaf tissue, expressing Bt in all plant tissues, controlling different generations of ECB, and herbicide tolerance.
Agbios. 2004. http://www.agbios.com/dbase.php
Biopesticides Registration Action Document: Bt Plant-Incorporated Protectants United States Environmental Protection Agency. October 15, 2001. http://www.epa.gov/oppbppd1/biopesticides/pips/bt_brad2/1-overview.pdf
Deacon, Jim. 2001. The Microbial World: Bacillus thuringiensis. University of Edinburgh, UK. http://helios.bto.ed.ac.uk/bto/microbes/bt.htm
Hoffmann, M.P. and A.C. Frodsham. 1993. Natural Enemies of Vegetable Insect Pests. Cooperative Extension, Cornell University. Ithaca, NY. 63 pp. http://www.nysaes.cornell.edu/ent/biocontrol/pathogens/bacteria.html
Holt, John G., and Peter H.A. Sheath., eds. 1984. Bergey’s Manual of Systematic Bacteriology New York. vol. 2, 1106-114, 1135 pp.
Hunt, T.E. and G.W. Echtenkamp. 2002. Resistance Management for European Corn Borer and Bt Transgenic Corn: Refuge Design and Placement. Nebraska Cooperative Extension NebFacts NF00-425. http://ianrpubs.unl.edu/insects/nf425.htm
Kelly Registration Systems. 2004. http://www.kellysolutions.com/ne/searchbyproductname.asp
Losey, J.E., L.S. Rayor, and M.E. Carter. 1999. Transgenic pollen harms monarch larvae. Nature. 399, pp 214.
Madigan, Michael T., John M. Martinko, and Jack Parker. 2000. Brock Biology of Microorganisms. 9th ed. New Jersey: Prentice-Hall Inc. 508-509 pp.
Mason, C.E., M.E. Rice, D.D. Calvin, J.W. Van Duyn, W.B. Showers, W.D. Hutchison, J.F. Witkowski, R.A. Higgins, D.W. Onstad, and G.P. Dively. 1996. European Corn Borer: Ecology and Management. Ames: Iowa State University, Pub. 327 North Central Regional Extension Publication.
Mendelsohn, M., J. Kough, Z. Vaituzis, and K. Matthews. 2003. Are Bt Crops Safe? Nature Biotechnology. 21:9. http://www.epa.gov/pesticides/biopesticides/pips/are_bt_crops_safe.pdf
Michigan State University Extension, Vanburen County IPM. 1997. http://www.msue.msu.edu/vanburen/ecb3.htm
National Corn Growers Association, Know Before You Grow. 2005. http://www.ncga.com/biotechnology/Search_hybrids/know_where.asp
Ramirez-Rocha, J. and T.M. Hurley. 2004. Who is Planting GM Crops and Why? The Thoughts and Experiences of Minnesota Farmers. University of Minnesota. http://www.apec.umn.edu/faculty/thurley/Extension/GMO2001SummaryResults.pdf
Reardon, R., N. Dubois, and W. McLane. 1994. Bacillus thuringiensis for Managing Gypsy Moth: A review. United States Department of Agriculture, Forest Service. FHM-NC-01-94. http://www.fs.fed.us/na/morgantown/fhp/gmoth/gm_news47/art2.htm
Schneider, K.R. 2002. Genetically Modified Organisms. University of Florida Cooperative Extension. FSHN02-2. http://edis.ifas.ufl.edu/pdffiles/FS/FS08400.pdf
Segarra, A.E. and J.M. Rawson. 2001. StarLink Corn Controversy: Background. CRS Report for Congress. http://www.ncseonline.org/nle/crsreports/agriculture/ag-101.cfm
Sloderbeck, Phil. 2004. Current Status of Bt Corn Hybrids. Kansas State Research and Extension. Manhattan: Kansas State University. http://www.oznet.ksu.edu/swao/Entomology/Bt_Folder/Bt%20Corns.html
1Transgenic Crops: An Introduction and Resource Guide. Colorado State University. 2004. http://www.colostate.edu/programs/lifesciences/TransgenicCrops/antibiosoil.html
2Transgenic Crops: An Introduction and Resource Guide. Colorado State University. 2004. http://www.colostate.edu/programs/lifesciences/TransgenicCrops/current.html#Bt
3Transgenic Crops: An Introduction and Resource Guide. Colorado State University. 2004. http://www.colostate.edu/programs/lifesciences/TransgenicCrops/hotmonarch.html
4Transgenic Crops: An Introduction and Resource Guide. Colorado State University. 2004. http://www.colostate.edu/programs/lifesciences/TransgenicCrops/soilleak.html
Willie, Bonnie. Accessed: July, 2001. The Natural Insecticide. http://gardenline.usask.ca/pests/bt.html
Witkowski, J.F., J.L. Wedberg, K.L. Steffey, P.E. Sloderbeck, B.D. Siegfried, M.E. Rice, C.D. Pilcher, D.W. Onstad, C.E. Mason, L.C. Lewis, D.A. Landis, A.J. Keaster, F. Huang, R.A. Higgins, M.J. Haas, M.E. Gray, K.L. Giles, J.E. Foster, P.M. Davis, D.D. Calvin, L.L. Buschman, P.C. Bolin, B.D. Barry, D.A. Andow & D.N. Alstad. 1997. Bt Corn & European Corn Borer: Long-Term Success Through Resistance Management. In K.R. Ostlie, W.D. Hutchison, & R. L. Hellmich eds. University of Minnesota Cooperative Extension. BU-07055-GO. http://www.extension.umn.edu/distribution/cropsystems/DC7055.html
Witkowski, John and Robert Wright. 1997. The European Corn Borer: Biology & Management. http://entomology.unl.edu/ecb/ecb1.htm#Item1
1Wright, R.J., S.D. Danielson, J.F. Witkowski, G.L. Hein, J.B. Campbell, K.J. Jarvi, R.C. Seymour, and J.A. Kalisch. 1994. Insect Management Guide for Nebraska Corn and Sorghum. Nebraska Cooperative Extension. Lincoln: University of Nebraska. EC94-1509-D. 13-18pp.
2Wright, R.J., T.E. Hunt, J.F. Witkowski, B.D. Siegfried, and J.E. Foster. 2000. Choosing a Bt Transgenic Corn Hybrid. Nebraska Cooperative Extension. Lincoln: University of Nebraska. NebFacts NF00-409. http://www.ianr.unl.edu/pubs/fieldcrops/nf409.htm